Text-message lobbying of senior NICE staff by individuals at NHS England and the Royal College of Psychiatrists in days before ME/CFS guideline pause
On August 4 2021, an embargoed copy of the final NICE guideline for ME/CFS — already severely delayed — was distributed to registered stakeholders “for a final factual error check, to enable [stakeholders] to highlight to NICE any substantive errors, and to prepare for publication and implementation.” The official publication of the guideline was scheduled for August 18.
However, on August 17, less than 12 hours before the scheduled publication time, NICE released a statement announcing that the guideline was being “paused”, citing “issues raised during the pre-publication period with the final guideline.” According to the statement, “unless the recommendations in the guideline are supported and implemented by professionals and the NHS, people with ME/CFS may not get the care and help they need.” On August 27, NICE released a further statement announcing that it was to “hold a roundtable event to better understand the issues raised and determine how it can gain support for the guideline to ensure effective implementation.”
Aware of the significant resistance to the draft guideline from certain quarters, as well as the resignations of three members of the guideline committee announced in early August, and wanting to understand exactly what had transpired leading up to the decision by NICE to pause the guideline, on August 17 I submitted a FOI request to NICE.
I am asking that NICE provides any and all (i) internal correspondence/communication between NICE staff (including that involving the guideline committee or any individual member(s) of the committee); and (ii) external correspondence/communication with professional bodies or individuals (guideline stakeholders or otherwise);
that details or relates to the the decision to pause publication of the final guideline and any justification given for the pause. This should include - but is not limited to - evidence of the issues and concerns raised during the pre-publication period with the final guideline, and NICE’s responses to these issues/concerns.
Please include all correspondence pertaining to the above stated matters between 29 March 2021 and 17 August, 2021. My request is not limited to only the pre-publication period (4–18 August 2021) as the decision to pause may have been influenced by opposition to the guideline or other issues/concerns registered before the pre-publication period.
I received a delayed response to my request on October 29, just hours after the guideline was finally published. On November 1, I requested that NICE conduct an internal review of its decisions, and, with some decisions overturned, on November 15 I obtained an updated set of documents from NICE that I am making available here, along with a readme file and a copy of my internal review request and the response from NICE.
Concerns and lack of support from royal colleges
The documents obtained reveal that on August 6, just two days after the release of the embargoed final guideline to stakeholders, an individual writing on behalf of the Royal College of Psychiatrists emailed Paul Chrisp — Director of the Centre for Guidelines at NICE — to formally let NICE know that the organisation would not be able to support the final guideline as they “continue to have major concerns about this guidance” (Document 2).
On August 9, Paul Chrisp reached out to an individual at the Royal College of Physicians to obtain their perspective on the guideline (Document 5). Based on information in this email conversation, this individual is likely to be Professor Lynne Turner-Stokes, author of a December 2020 editorial in the BMJ that criticised the draft guideline and, in particular, NICE’s approach to evidence evaluation. Responding on August 13, this individual asks Paul Chrisp if there is “actually any chance that anything we might say could still change things at this stage or is it simply a case of responding after publication?”, before Paul Chrisp suggests the two have a phone call. It is not clear if this conversation took place, and, if so, how it proceeded.
“There is a way.”
Meanwhile, on August 9, the Chief Executive of NICE, Gillian Leng, contacted Paul Chrisp via text (Text exchange 1) to request the two have a phone call. This seems to have been prompted by Gillian’s conversation earlier that day with an individual whose name has been redacted. It seems that after the phone call between Gillian and Paul, Gillian forwarded a copied and pasted message she received from a stakeholder. The message (split between pages 2 and 3 of Text exchange 1) is shown below. Note that this image has been created so that the message is shown as a whole, but none of the text has been changed.
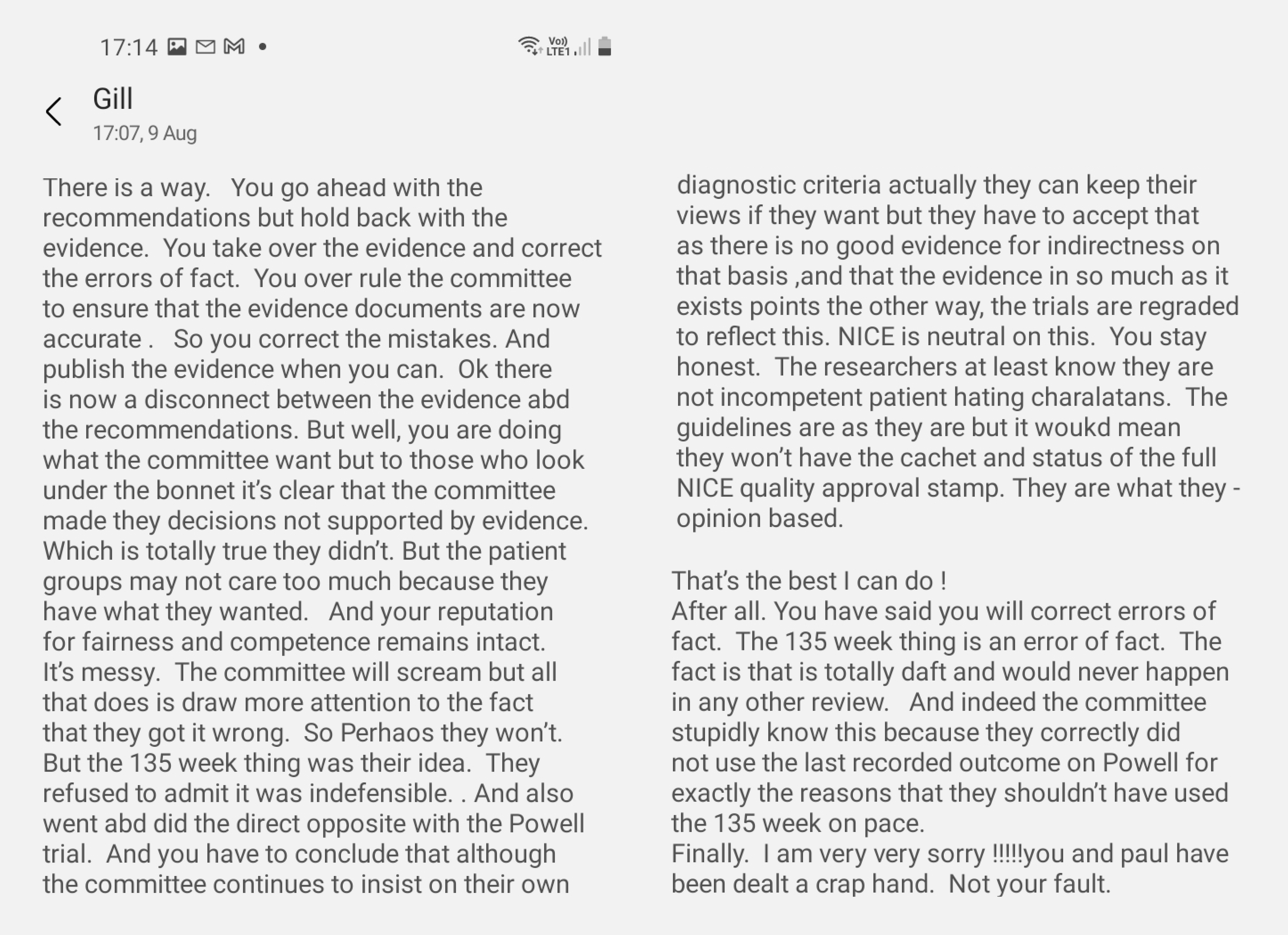
Those unfamiliar with the NICE process might have trouble understanding what exactly this individual is proposing (to none other than the Chief Executive of NICE). The individual is not particularly concerned with the guideline recommendations per se. Rather, the author of the message insists that NICE overrides and tampers with the Evidence Review — which forms the basis of the guideline recommendations — to reverse the decision to downgrade the quality of evidence from trials such as PACE (or “pace” as this individual casually refers to it.). The references to “Powell” and “the 135 week thing” refer respectively to the decision by the guideline committee not to include a particular trial (Powell et al, 2001), and the NICE protocol that mandates that trial data are analysed using the longest follow-up data available, rather than data at the pre-specified endpoint for the primary outcome measures.
This is clearly a highly-incriminating message that should never have been sent to Gillian. One can only assume that this is a high-placed individual, familiar with the intricacies of the trial data, personally known to Gillian, who has no qualms about suggesting such action after the final guideline had been signed off and distributed to stakeholders.
After this message is forwarded, Paul responds to Gillian saying “I’ve run this idea past [REDACTED], he will pick up with [REDACTED] and get back to me tomorrow”. When the text conversation continues on August 13, it is clear that discussions of the practicalities and implications of a delay/postponement have taken place among senior staff at NICE. Paul then shares with Gillian some “reflections” — either his own, or those of an unknown party whose details have been redacted. These include: (i) the possible need for legal advice, (ii) the implications of withdrawing the 2007 guideline, and (iii) possible “unintended consequences for other guidelines on complex conditions that have attracted controversy, long COVID, chronic pain.”
One of the key reasons for my requesting an internal review was to find out the organisational identity of the individual who sent this message to Gillian, as this information was not included in the response to my initial request. In response to my internal review request, NICE confirmed that this individual “is associated with the Royal College of Psychiatrists.” This came as no surprise to me; I had my own ideas about who this individual is, and we extensively discussed the FOI material — particularly this message — on the S4ME forum, with many coming to the same conclusion. Note that the personal data (such as names and contact details) relating to third parties (in this case individuals representing guideline stakeholders) are usually considered exempt from disclosure under section 40 of the FOI act. I did not challenge this decision in my request for an internal review.
“The least worse option is to delay or pull it altogether”
On 11 August, Paul reached out via text message (Text exchange 2) to an individual at NHS England & NHS Improvement (NHSE&I), asking if they’ve seen the embargoed guideline, and suggesting they talk on the phone. With a phone conversation likely having taken place that afternoon, Paul messages this individual back saying “we are considering our options, more next week”. This is followed by a message from the individual at NHSE&I suggesting that “the least worst option is to delay it or pull it altogether.” The final two messages of Text exchange 2 are shown below.
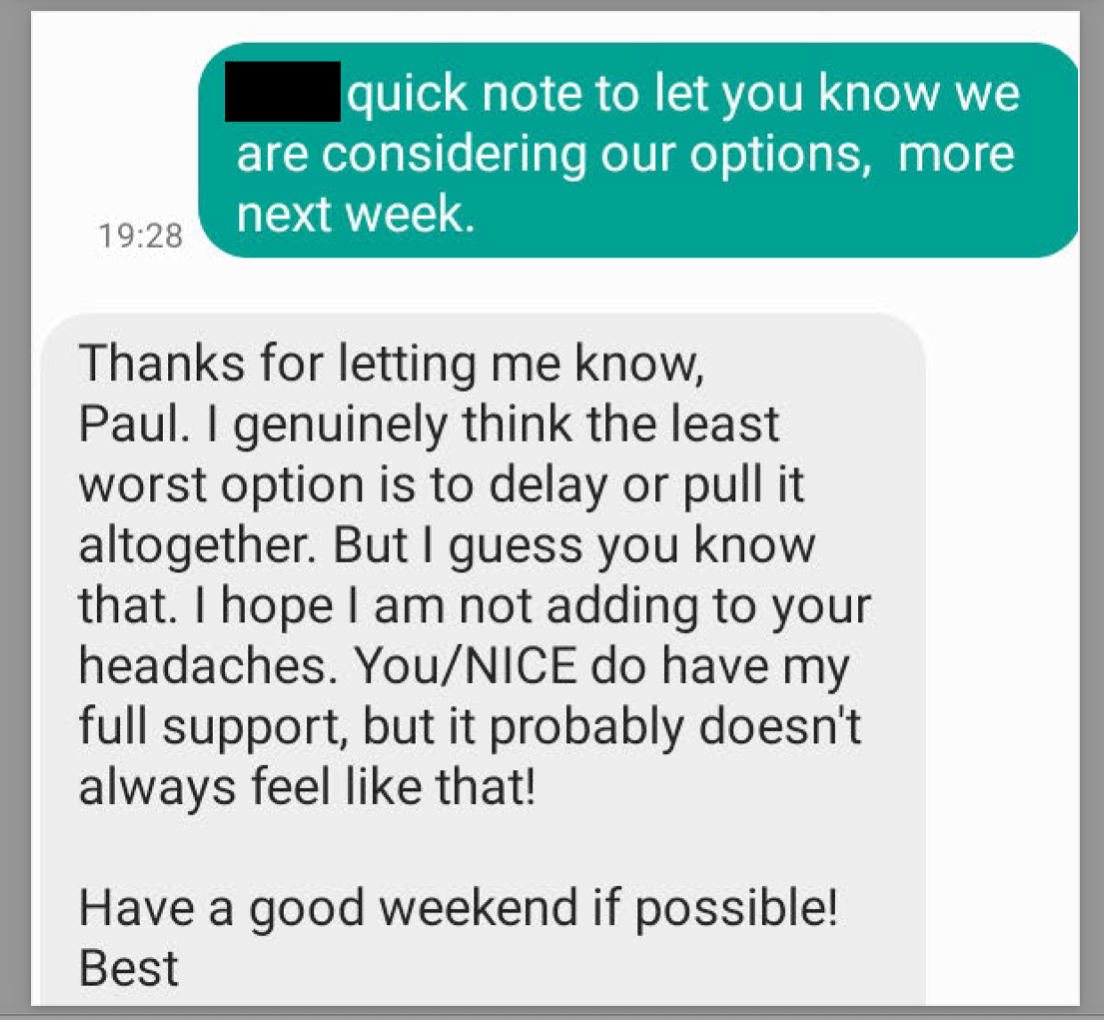
This individual then emails Paul later that evening (Document 4). We know from the response to my initial FOI request that “Text exchange 2 is a between the same parties as document 4.” In their email, this individual makes similar claims as those made by the author of the copied and pasted message in Text exchange 1 — namely that trials have been excluded on spurious grounds, and that the guideline is now opinion-based rather than evidence-based — they are additionally concerned about the optics of NICE removing so-called treatments and offering nothing new in their place, and the potential damage to NICE’s reputation, though this seems to be written from a place of strategy and persuasion as much as it was genuine concern for NICE. The email finishes with the author reiterating their call for delay/postponement of the guideline.
The opposition from NHSE&I to the guideline was not new. When the draft guideline was published in December 2020 for stakeholder consultation, the official stakeholder response comments from NHSE&I were accompanied by a letter written to the Clinical Programmes team at NHSE&I by Professor Tim Kendall, a consultant psychiatrist, Director of the National Collaborating Centre for Mental Health (NCCMH), and the National Clinical Director for Mental Heath at NHSE&I. This letter, available here, was part of a set of documents obtained from a FOI request to NHSE&I by a member of S4ME (and only after involvement of the Information Commissioner’s Office). The documents were originally released with identities redacted. However, as the author of this letter was made known in an accompanying email thread, I am providing a version of the letter with redactions removed — only possible due to NHSE&I’s ineptitude.
The letter is a scathing but deeply flawed assessment of the draft guideline, claiming that the guideline is no longer about the illness ME/CFS, but rather about “PESE”, referring to the guideline committee’s decision to use only diagnostic criteria that include post-exertional symptom exacerbation (more commonly referred to as post-exertional malaise (PEM)). The letter also takes aim at the guideline committee, who Prof. Kendall charges with bias against “psychology or psychiatry in general”.
At this point I should say that it is not clear whether Prof. Kendall is the author of the email in Document 4 and the person with whom Paul Chrisp is communicating with in Text exchange 2, as the personal identity of this individual has not been revealed.
The remaining documents (documents 1, 3, and 6) reveal that the decision not to publish the guideline on August 18 was made on or shortly before August 16 (Document 3); the decision to ‘pause’ the guideline was signed-off on August 17 at the Guideline Executive meeting (Document 1); and that there had been discussions between Paul Chrisp and a Policy Officer at the Department for Health and Social Care the prior day (Document 6) in which a pause is considered one possible option.
The end of the beginning
Given the favourable outcome of the subsequent roundtable discussion on October 18 — which brought together the professional groups who had expressed their lack of support of the guideline with representatives of patient charities and advocacy groups — one might be minded to consign this prolonged and immensely frustrating saga to the history books. In my opinion, to do so would be extremely foolish and naive.
First, opposition to the updated ME/CFS guideline has not miraculously dissipated with its publication. Comments in the media from the likes of Prof. Peter White and Dr Alastair Miller show that the old guard is not prepared to give up the ghost. Just last week, the biopsychosocial cult’s newest torchbearer, Prof. Paul Garner, again took to Twitter to bait patients with articles from Norway critical of the guideline and NICE’s methodology, and claiming corruption of the process and patient “activism”. The irony is that the activism and lobbying was not from patients but rather from those in high places with vested interests, their own biases, and a direct line to NICE executives. The publication of the guideline marks the end of the beginning, rather than the beginning of the end, and we should be prepared for further opposition and dragging of feet as the guideline is implemented.
Second, there are questions about how widespread such interference is in the NICE guideline process. Have key decisions around other guidelines been influenced? The recent FOI requests to NICE and NHSE&I have turned up exactly the sort of meddling I thought might be occurring behind the scenes. There is a designated channel via which stakeholders partake in the guideline development process. And whilst it might be expected that NICE consult key stakeholders in the case of contested guidelines, fielding proposals from individuals on how to proceed with an already-signed-off guideline should not be part of the process.
I believe that patients and other stakeholders — who played by the rules throughout the process — deserve to know the identity of the individual “associated with the Royal College of Psychiatrists” who sent the copied and pasted message shown above. I believe the Royal College of Psychiatrists should be approached to find out just how much they knew about such back channel correspondence, and I also believe that NICE has some questions to answer about how they dealt with this proposal and indeed how they separate legitimate concerns from stakeholders from text-message lobbying.
I’d like to end this blog by thanking everyone who worked so hard on guideline, and those who pressed to make sure it was published: this includes those who systematically interrogated the evidence base and collected evidence of patient harm; the many stakeholders who submitted excellent comments on the scoping documents and the draft guideline; those who represented patients at the roundtable meeting; the Guideline Committee, who did a great job; and NICE itself, who, with obvious exceptions, maintained the rigorous process and standards for which it is known. Finally, I’d like to thank all those on the S4ME forum who helped make sense of all the FOI documents and the timeline of events, and those who commented on a draft version of this blog.
Leave a comment